Biostable, durable and customizable medical polyurethanes
Our strong, durable, yet flexible medical polyurethane portfolio is broad and versatile – ideal for load-bearing implantable devices where excellent biostability and long-term performance is essential. These unique biomaterials also give you exceptional versatility. They can be customized and combined with a wide range of materials to bring you even greater performance.
We work with partners worldwide across the continuum of healthcare to create innovative medical devices that bring progress to life for patients everywhere
30+
Years
30+ years of proven clinical use.
200k+
200k+ spinal implants annually.
14+
Million
14 million+ cardiac and neurostimulation leads annually.
Why choose our medical grade polyurethanes for your next project?
Specifically designed for long-term implantation in the human body, our medical polyurethanes are ideal for demanding applications like soft tissue augmentation, cartilage repair, and resurfacing implants – as well as glycemic control devices for diabetic patients.
They also bring you further versatility in device design, through tunable bulk properties and surface properties achieved using Surface Modifying End Group (SME®) technology. Complementing all this we bring you added peace of mind through:
- Extensive FDA Master Files for each family of polyurethanes that support US regulatory filings.
- Specialized production capabilities based on an established supply chain; robust quality systems; and a medical grade, ISO-certified manufacturing facility.
Portfolio Overview
Discover our solutions for medical grade polyurethanes
- Bionate®
- Bionate® II
- CarboSil®
- Biomerix
- Elasthane®
- ATPU
- Biospan®
- PurSil®
Bionate®
Our aromatic polycarbonate-based thermoplastic polyurethanes (PCUs) are tough, with excellent oxidative biostability and abrasion resistance; making them ideal for use in:
- Load-bearing and articulating implants.
- Long-term orthopedic applications.
- Lead insulation applications: Bionate® has excellent dielectric properties.
Bionate® II
These medical polyurethanes give you similar polymer performance to Bionate® - but enhanced with SME® technology, which modifies the surface characteristics to improve manufacturing.
CarboSil®
Our aromatic polycarbonate-based silicone-containing thermoplastic polyurethanes (TSPCUs) combine the biocompatibility and biostability of conventional silicone elastomers with the processability and toughness of thermoplastic polycarbonate-urethanes. This makes them:
- Ideal for use in cardiovascular or orthopedic implantable devices.
- Proven in applications ranging from continuous glucose monitoring devices to analyte sensors and neuromodulation devices.
Biomerix
Our aromatic polycarbonate-based thermoset polyurethane (PCPU) provides a unique scaffold with an open-cell, porous structure. This is complemented by:
- Varying compressibility with high void content (90%-95% void area).
- Availability in various shapes and forms; and customization based on your application requirements.
Elasthane®
Our aromatic polyether-based thermoplastic polyurethane (TPU) is a durable and abrasion-resistant, workhorse polymer with a 20-year track-record in chronic implants.
Ideal for insulating sheathing in pacing leads (CRM).
ATPU
For applications that require low processing temperatures, our aliphatic polyether-based thermoplastic polyurethane (ATPU) is the ideal choice, offering:
- A combination of high strength and elongation in temporary implant applications.
- A proven track record in a variety of applications; for example, as an excipient platform for hydrophobic and hydrophilic drug diffusion.
Biospan® (SPU)
Our segmented polyether polyurethane (SPU) is delivered in a dimethylacetamide solution. It enables extraordinary flex life and is proven to withstand millions of flex cycles.
- Biospan® contains two individual formulations - enhanced with silicone or fluorocarbon surface-modifying end group technology.
- Ideal for cardiovascular applications: including ventricular assist, artificial hearts, and balloon devices.
PurSil® (TSPU)
For the best of both silicone elastomers and polyether urethanes, look no further than our aromatic polyether-based silicone-containing thermoplastic polyurethane (TSPU).
- PurSil® delivers excellent flexibility and oxidative stability.
- Ideal for various medical device applications: including ophthalmic and continuous glucose monitoring.
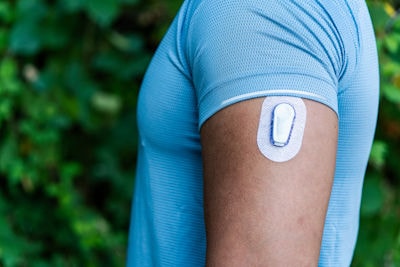
Application spotlight: Next-level glycemic control
Sparsa is a next-generation biomaterial developed by our experts to bring new levels of performance to glucose-limiting membranes.
This unique, amphiphilic polyurethane enables both oxygen and glucose to diffuse through the bio-sensors - and deliver fast, accurate readings using a single material. The result is greater efficiency and sustainability for our partners – with less materials required and no need for polymer multi-blends.
It’s good news for patients too. Sparsa is not just limited to monitoring glucose. It can also be used to deliver accurate readings for various other analytes throughout the day – thus enabling diabetes sufferers to manage their health even more effectively.
We create sustainable healthcare solutions that deliver tangible and valuable sustainability benefits to our partners – with no compromises on safety or performance.
Read the full Med Device Online article.
Learn about our solutions for Diabetes.
Frequently Asked Questions
Here are some common questions our experts get asked. If you have a different question, please contact us.
In what forms are your medical polyurethanes available?
These thermoplastic polymers are supplied as pellets; but also come in solution form. Please contact your DSM representative to learn more.
How should the material be stored?
Store our polyurethanes at 15-32°C (60-90°F) in a relatively dry, dark place (due to the hygroscopic nature of this material). If possible, the entire contents of a container should be used at one time. If this isn’t possible, you should put the remainder in a re-sealable container - ideally blanketed with an inert gas (such as dry nitrogen).
How should your medical polyurethanes be processed?
Our polyurethanes can be processed using many thermoplastic methods: including injection molding, extrusion and compression molding. Melt processing conditions are similar to conventional thermoplastic polyurethanes. For more information, download our TPU Extrusion Guide and/or The DSM Injection Molding Guide.
- Filtered solutions of the thermoplastic polymers are suitable for solvent bonding, dipping, coating, spraying, and related solvent-based conversion methods. For more information, contact our team of experts.
How should the polyurethanes be dried before thermal processing?
Proper drying of the pellets is an important first step to ensure efficient thermoplastic processing and the production of high-quality parts. A desiccant-bed-type dehumidifying hopper dryer is recommended, with an inlet air temperature of 80o – 95o C (180o – 200o F) for Bionate®, Bionate® II, CarboSil®, Elasthane®, and PurSil® polyurethanes. For ATPU grades the inlet air temperature should be set to 60o – 80o C (140o – 176o F).
- Drying to approximately 0.01-0.02% moisture can usually be accomplished in 8 to 12 hours. However, the time will depend on the efficiency of your dryer and the amount of material to be dried. The harder durometer materials (e.g. 55D & 75D) may need a drying temperature and time at the higher end of the recommended range.
- The moisture content of medical polyurethanes should be measured by a moisture analyzer immediately before each use. After drying, the material may need to be manually separated (using a gloved hand) before melt processing in order to prevent the throat of the extrusion or injection molding equipment becoming blocked. For injection molding, a dew point reading of approximately -20 to -40°C (-5 to -40°F) on the return air hopper is also a good indicator that the material is ready for processing. Note: We do not recommend over-drying or re-drying polyurethanes as this may cause sub-optimum processing and gels.
How do you sterilize medical polyurethanes?
We recommend Ethylene Oxide (EtO) for sterilizing devices made with polyurethanes, based on published references. Our testing indicated that EtO sterilization has no noticeable effect on the mechanical properties of polyurethane parts. Gamma radiation is another sterilization method for parts made from our medical polyurethanes. In fact, when tested, polyurethanes from DSM Biomedical showed no changes in mechanical properties when sterilized by gamma irradiation up to 45 KGray (1 Gray= 1 Joule/Kg). We do not recommend using steam sterilization; although other methods of sterilization may be appropriate, depending on the design of the part. Ultimately, device manufacturers are responsible for verifying the impact of the sterilization process on their products’ performance.
Technical Resources
Looking for more information on how our ECM technology can help you tackle unmet needs?
ATPU Product Sheet
Bionate® PCU Product Sheet
Bionate® II PCU Product Sheet
BioSpan® SPU Product Sheet
CarboSil® TSPCU Product Sheet
PurSil® TSPU Product Sheet
We understand that medical product development can be a long, winding - and occasionally bumpy road. Rest assured that at DSM Biomedical, we have the proven experience, capabilities, and knowledge to support you every step of the way.
It starts with using a form-fit-function approach based on a comprehensive understanding of how the human body reacts to biomaterials following implantation. This in turn enables us to design materials that are compatible with the body’s physiology and help you develop finished products that can sustain, restore, and repair – all supported by applicable regulatory requirements.
MDSAP ISO 13485
Our expert biomaterials team is here and ready to support you
Discover how we can partner with you to create innovative medical devices with our medical polyurethanes.
Need expert assistance?
Our polyurethane experts are here to help.